
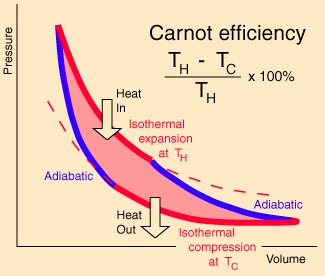
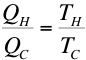

 The above expression applies
specifically to the Carnot cycle. It can be
shown that any
reversible cycle can be described as a sequence of
infinitesimal Carnot cycles (see figure at
right). In this case the summation above
becomes an integral
The above expression applies
specifically to the Carnot cycle. It can be
shown that any
reversible cycle can be described as a sequence of
infinitesimal Carnot cycles (see figure at
right). In this case the summation above
becomes an integral


 Since entropy is
unchanged in a complete cycle it is a
"state" variable. Other state
variables we have encountered include:
pressure, temperature, volume, internal
energy, gravitational potential
energy. All these variables have
specific values in one configuration of a
system which are retained if the system
leaves then returns to its initial
configuration.
Since entropy is
unchanged in a complete cycle it is a
"state" variable. Other state
variables we have encountered include:
pressure, temperature, volume, internal
energy, gravitational potential
energy. All these variables have
specific values in one configuration of a
system which are retained if the system
leaves then returns to its initial
configuration. If the system in
question does not undergo a complete
cycle then
If the system in
question does not undergo a complete
cycle then
 is independent
of the path. Therefore,
whether the path is reversible or
irreversible the entropy change will
be the same and we can use the above
equation to calculate these entropy
changes.
is independent
of the path. Therefore,
whether the path is reversible or
irreversible the entropy change will
be the same and we can use the above
equation to calculate these entropy
changes.

 The
concept
of entropy isn't limited
to thermodynamic systems.
In fact, via statistical
mechanics, it can be shown that
entropy is related to the disorder
in a system. The
second law then states that "All
allowed processes take place
such that the disorder of the
Universe increases".
The
concept
of entropy isn't limited
to thermodynamic systems.
In fact, via statistical
mechanics, it can be shown that
entropy is related to the disorder
in a system. The
second law then states that "All
allowed processes take place
such that the disorder of the
Universe increases". Even
more general still, it appears
that the "arrow
of time", the fact
that time always flows
in one direction (past to future),
is defined by the necessity of the
entropy of the Universe to
increase.
Even
more general still, it appears
that the "arrow
of time", the fact
that time always flows
in one direction (past to future),
is defined by the necessity of the
entropy of the Universe to
increase.
![]()
Have you
heard that entropy isn't what it used to be?
Dr. C. L. Davis
Physics Department
University of Louisville
email: c.l.davis@louisville.edu
