
Coulomb's Law

"When man wanted to make a machine that would
walk
he created the wheel, which does not resemble a leg"
Guillaume Apollinaire

- The magnitude of the force of attraction (or repulsion), F12
between two point charges q1 and q 2 is
given by Coulomb's Law.
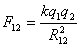
where R12 is the distance between the
charges. k is a constant of proportionality, having the value 9 x
109 N.m2 / C2 in a vacuum.
- The direction of this force is along the line joining the two
charges
with the sense determined by the relative signs of the charges
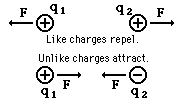
- Note that the force on each charge has the same magnitude (as
required by Newton's third law of motion).
- For two 1 Coulomb charges separated by 1 metre the
magnitude
of the force is given by,
F = (9 x 109 x 1 x 1 )/ 1 = 9 x 109
Newtons
This is an extremely large force (sufficient to
move Mt. Everest with an acceleration of 1cm/s2). The
Coulomb
is a very large unit. Typical macroscopic charges
are measured in micro-coulombs (10-6 C).

"The
wireless telegraph is not difficult to
understand. The ordinary telegraph is like a very long cat. You pull
the tail
in New York,
and it meows in Los Angeles. The wireless is
the same, only without the cat."
Albert Einstein

Dr. C. L. Davis
Physics Department
University of Louisville
email: c.l.davis@louisville.edu


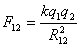
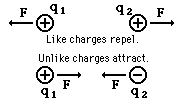
![]()
