
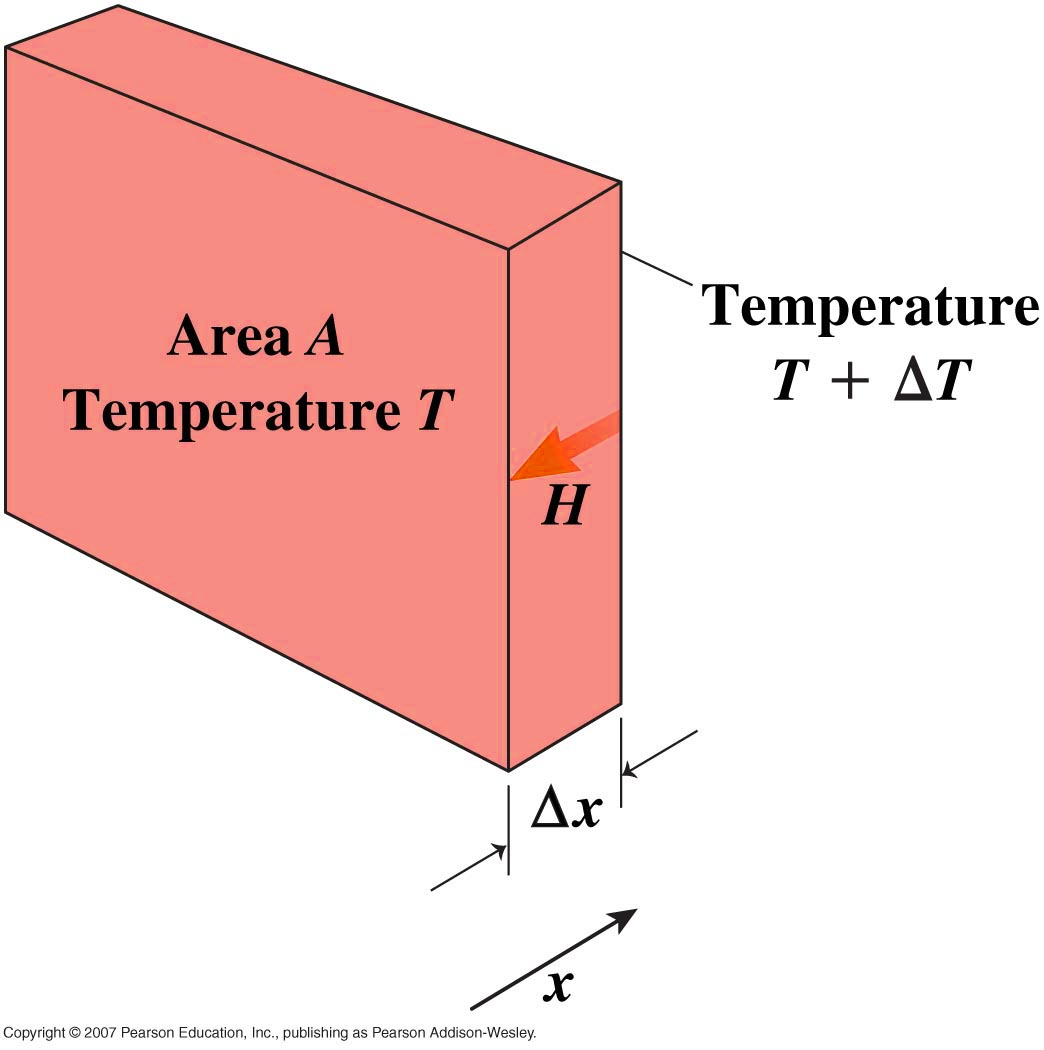
 For a rod, cross section
A and length
For a rod, cross section
A and length 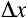 experimentally we find that
the rate of heat transfer (dQ/dt) is given by,
experimentally we find that
the rate of heat transfer (dQ/dt) is given by,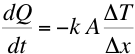
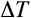 is the change in temperature and k is the thermal conductivity of the
material, measured in Watts/m.0C.
is the change in temperature and k is the thermal conductivity of the
material, measured in Watts/m.0C.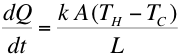
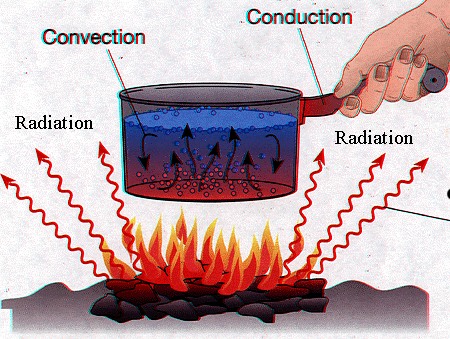
 Caused by the macroscopic motion of
the substance, only possible when the atoms/molecules
can migrate through the sample as in gases and liquids
(fluids).
Caused by the macroscopic motion of
the substance, only possible when the atoms/molecules
can migrate through the sample as in gases and liquids
(fluids).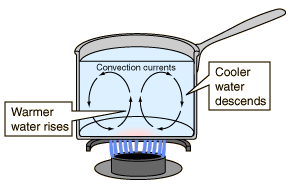
![]()
You enter
the laboratory and see an experiment.
How will you know which class is it?
If it's green and wiggles, it's biology.
If it stinks, it's chemistry.
If it doesn't work, it's physics.
Dr. C. L. Davis
Physics Department
University of Louisville
email: c.l.davis@louisville.edu
