

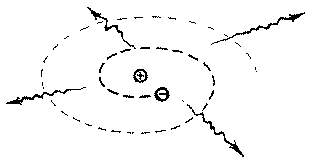
 Particles moving in orbits are undergoing
circular motion. A particle undergoing circular motion has a
continuously changing velocity. A particle with a continuously
changing velocity is being accelerated. Therefore the orbitting
electron feels an acceleration and must lose (radiate) energy.
As a result of losing energy the electron will gradually spiral
down onto the proton. Calculations indicate that this
"gradual" spiral would take a fraction of a second.
Particles moving in orbits are undergoing
circular motion. A particle undergoing circular motion has a
continuously changing velocity. A particle with a continuously
changing velocity is being accelerated. Therefore the orbitting
electron feels an acceleration and must lose (radiate) energy.
As a result of losing energy the electron will gradually spiral
down onto the proton. Calculations indicate that this
"gradual" spiral would take a fraction of a second. 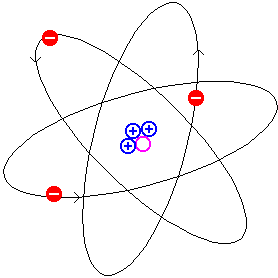 becomes
becomes 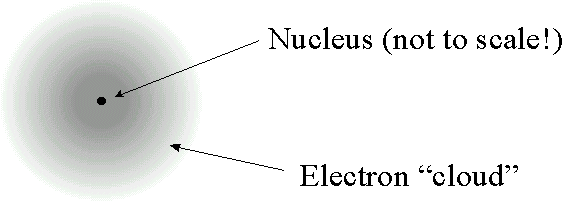


Dr. C. L. Davis
Physics Department
University of Louisville
email: c.l.davis@louisville.edu
